How to Determine Which Resonance Structure Is Best
Whenever you can draw two or more Lewis structures for a molecule differing only in the locations of the electrons the actual structure is none of the structures but is a hybrid of them all. C is technically a valid resonance structure.

How To Choose The More Stable Resonance Structure Chemistry Steps
A phenomenon in which an external force or a vibrating system forces another system around it to vibrate with greater amplitude at a specified frequency of operation.
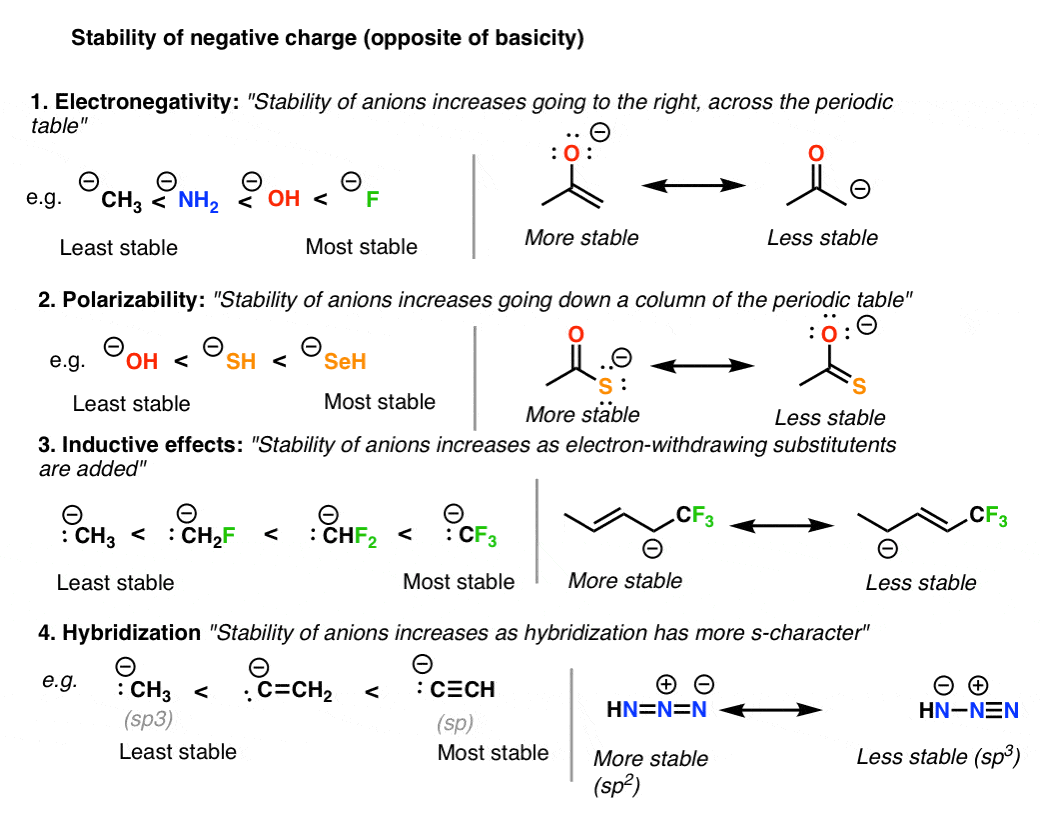
. 1 The same molecular formulas. A molecule can have resonance structures when it has a lone pair or a double bond on the atom next to a double bond. The resonance structure with a.
Determine which structure would be the best most stable and explain why in the box below. In these situations it is helpful to calculate the formal charge on each atom in each possible resonance structure and use the formal charges to determine the most representative structure. Ground rules for writing resonance structures.
This is important because neither resonance structure actually exists instead there is. The most stable structures are known as the major contributors. Formal charge Group number - number of nonbonding e-- number of bonding e- 2.
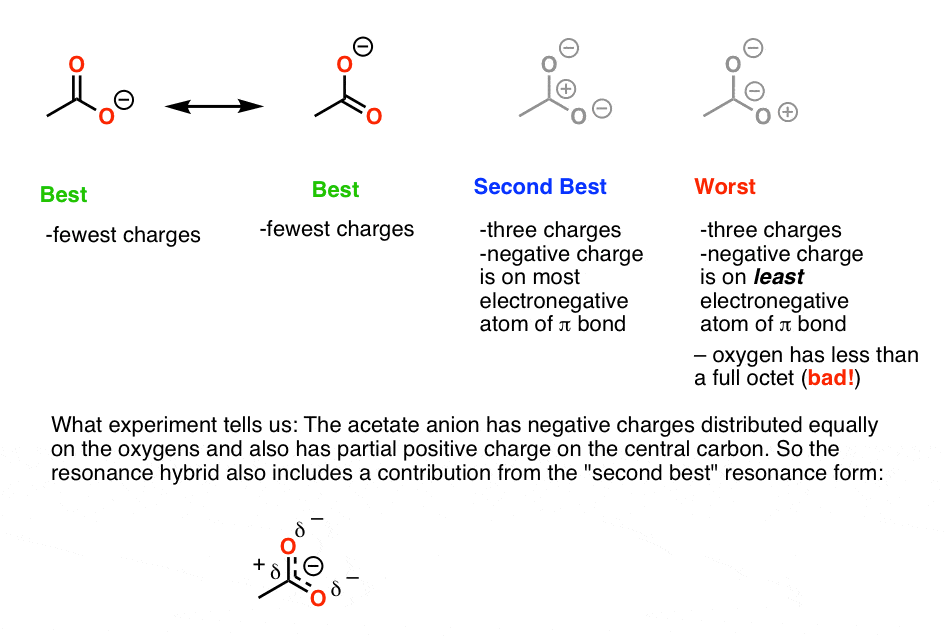
It has minimum charge separation ie. The two oxygens are both partially negative this is what the resonance structures tell you. This is significant because the greater the stability of a singular structure the more it will contribute to the resonance hybrid.
Resonance in physics is defined as follows. A resonance structure has a greater importance if. These structures are called resonance structures or contributing structures.
However it is important to note that each of these structures cannot actually be observed in nature. Explain in the box below. H 0N0 ô and H-öîö.
Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered. B Draw three resonance structures for HCO3 and include the formal charge on each atom. We divide the bonding electron pairs equally for all ICl bonds.
In these 2p_z orbitals there can be 0 1 or 2 electrons. Need help with chemistry. You have to compare S O X 2 and S O X 3 X because they distinguish A from B and C from D.
You can derive it from B by moving the electron pair from the double bond between the carbons 2 and 3 numbering from the left. That will normally be the least electronegative atom N. While both resonance structures are chemically identical the negative charge is on a different oxygen in each.
Rather the true structure is an approximate intermediate between each of the structures. We assign lone pairs of electrons to their atoms. 2 The same total number of electrons same overall charge.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Draw the Lewis Structure Resonance for the molecule using solid lines for bonds. Where there can be a double or triple bond draw a dotted line ----- for the bond.
Decide which is the central atom in the structure. The most stable resonance structure will have a full octet on every atom. Fewer non-zero formal charges 3.
This may not be correct from a nomenclature standpoint but Im using it for this answer onto carbon 2 and moving the electron pair from the double bond between the oxygen and the carbon onto. The most stable resonance structure will have negative charges on the most electronegative atoms and positive charges on the least electronegative atoms. That is the molecule does not actually go back and forth between these configurations.
Atomic positions and the skeleton of sigma bonds should remain the same in all resonance structures. 3 The same atoms connected together. In the example below we calculate the formal charge on each atom in a.
Although they can differ in whether the connections are single double or triple bonds. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Resonance structures with the lowest amount of energy are known to be the most stable.
It contains a greater number of bonds and atom octets 2. 7 7 0. My question is about resonance of Lewis structures.
Each Cl atom now has seven electrons assigned to it and the I atom has eight. 7 8 1. If it has only one Lewis structure it doesnt have a resonance hybrid.
In order to find the resonance structure that contributes the most you need to calculate the formal charge of each of the elements within the lewis structure and compare it to the formal charges on other resonance structures. How do you determine the most stable resonance structure. But the actual molecule has an intermediate structure of those possible Lewis structures.
Atoms in general dont like charges so having no charge is better. Not all resonance structures are equal there are some that are better than others. The most stable resonance structure will have the smallest possible number of charges.
But if multiple resonance structures. The negative charge resides on a more electronegative atom 4. Subtract this number from the number of valence electrons for the neutral atom.
Please draw your structures on a piece of paper and embed the picture directly in your post. Each contributing resonance structure can be visualized by drawing a Lewis structure. Preferred Resonance Structure of Molecule.
Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules. The better ones have minimal formal charges negative formal charges are the most electronegative atoms and bond is maximized in the structure. If a molecule has multiple resonance structures I understand that the preferred structureone that is the best contributor to the molecules resonance is the one with the lowest formal charges on each atom.
The one with the lowest and least number of formal charges other than 0 is the one that is the most favorable. The concept of resonance would be hard to. The first step of drawing resonance structures starts with drawing all the possible Lewis structures.
You have to compare N H X 3 and N O X 3 X because the distinguish A and B from C and D. No like charges are located on adjacent atoms. Draw only the lone pairs found in all resonance structures do not include the lone pairs that are not on all of the resonance structures.
If you use this strategy you miss out on the chance to figure out S O X 3 ethanol and benzene and you would learn less. All atoms should lay in the same plane with the 2p_z orbitals perpendicular to this plane.

Resonance Structures 4 Rules On How To Evaluate Them With Practice
How To Find The Best Resonance Structure By Applying Electronegativity

No comments for "How to Determine Which Resonance Structure Is Best"
Post a Comment